How to Determine the Medical Device Classification?

|
In this article, you will learn what is the Medical Device Classification and Classification Panels as per the Food, Drug, and Cosmetic Act (FD&C Act) and which considerations involve in terms of regulatory requirements. A medical device is a product, such as an instrument, machine, implant, or in vitro reagent, that is intended for use in the diagnosis, prevention, and treatment of diseases or other medical conditions. Read more information about what is a medical device according to the FD&C Act. FDA Video about Medical Device Classification.
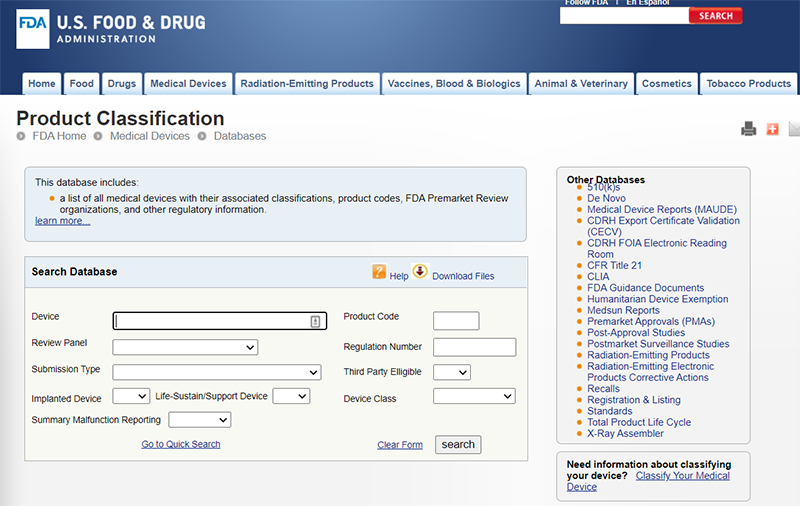
Determine your Medical Device ClassificationSearch the Product Classification DatabaseSearch the FDA Product Classification Database to determine if there is an existing product classification that applies to your product:
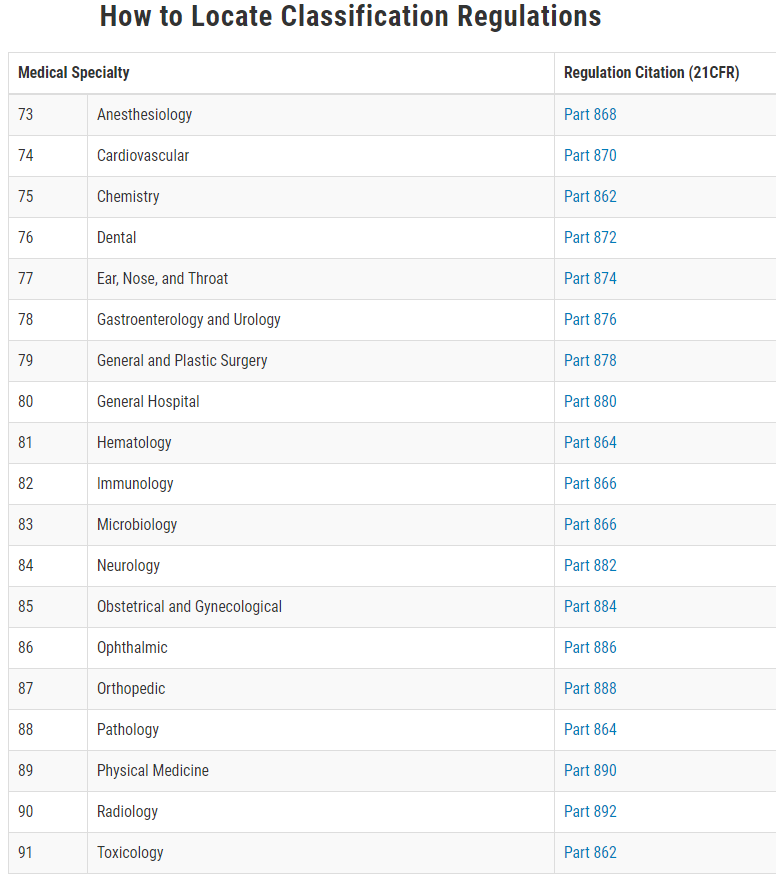
At this moment, the Food and Drug Administration (FDA) has defined classifications for approximately 1,700 different generic types of devices and grouped them into 16 medical specialties referred to as panels. What are the Medical Devices Classification PanelsTypically, the medical devices can be classified by finding the matching description of the device in Title 21 of the Code of Federal Regulations (CFR), Parts 862-892. In addition, the FDA has classified and described over 1,700 distinct types of devices and organized them in the CFR into 16 medical specialty “panels” such as Cardiovascular devices or Ear, Nose, and Throat Devices. These panels are found in Parts 862 through 892 in the CFR. For each of the devices classified by the FDA, the CFR gives a general description including the intended use, the class to which the device belongs (i.e., Class I, II, or III), and information about marketing requirements. Your device should meet the definition in a classification regulation contained in 21 CFR 862-892. Each of these generic types of devices is assigned to one of three regulatory classes based on the level of control necessary to assure the safety and effectiveness of the device. THE (3) THREE MEDICAL DEVICES CLASSIFICATIONS OR CLASSES:1. Class I General Controls
2. Class II General Controls and Special Controls
3. Class III General Controls and Premarket ApprovalMEDICAL DEVICES REQUIREMENTS BASED ON ITS CLASS.The class to which your device is assigned determines, among other things, the type of premarketing submission/application required for FDA clearance to market. If your device is classified as Class I or II, and if it is not exempt, a 510k will be required for marketing.All devices classified as exempt are subject to the limitations on exemptions. Limitations of device exemptions are covered under 21 CFR xxx.9, where xxx refers to Parts 862-892. For Class III devices, a premarket approval application (PMA) will be required. Otherwise, a 510k will be the route to the market if your device is a preamendments device (on the market prior to the passage of the medical device amendments in 1976, or substantially equivalent to such a device) and PMA’s have not been called for. For further information on how to classify a medical device, please refer to the Classify Your Device page. Read About How to Determine if Your Product is a Medical DeviceNeed help & support with your medical devices classification or need templates to create a quality manual and other procedures, visit us,SUBSCRIBE AND FOLLOW US TO LEARN MORE ABOUT MEDICAL DEVICES CLASSIFICATIONS PER FOOD, DRUG, AND COSMETIC ACT.For more details on specific FDA expectations and how to create quality management system documents necessary, follow us. Three (3) Options to Create Quality Management Procedures:Bronze Option. You Can Create Your Own Quality Procedures, using a Template.You can download samples of the quality procedure templates in .pdf format. To see the complete list of the most popular quality procedures templates, click here. In addition, you can request a quotation to buy online a full SOP template document in MS Word format that is completely editable, ready to fill and adapt to your specific needs. Silver Option. We Can Bring You a Formal Training on Medical Devices Classifications?This option is recommended if you want to learn more about how to build robust quality system procedures. One of our expert(s) can provide online step-by-step training to your team (unlimited assistance) on how to build reliable SOPs using our template(s). Also, you can improve your corporate quality procedures and policies by incorporating our template(s) and tools. It includes a fully editable template from the Bronze option, plus training, exams, and a training certificate for each assistant. Request a quote now. Gold Option. We Can Create Customized Quality Management Procedures.One of our expert(s) will create and prepare your customized SOPs with the inputs and specific information of your company. It includes a fully editable template from the Bronze option, plus online support in document creation, implementation, and training. Request a quote online. GET IN COMPLIANCE TODAY, CONTACT US (Hablamos Español)REFERENCES:For more information about Medical Devices Classification, refer to:
Further AssistanceDivision of Industry and Consumer Education (DICE). |

Ramon Cayuela, MS, BS, Chemical Engineering
CIQA President and CEO.
I've been working in validation engineering since 1992 with many multinational pharmaceutical companies. I love sharing my passion and knowledge with others. If you have any questions about anything (or just have general questions). I will be more than happy to assist you. You can count on the BEST customer service on CIQA. I go to great lengths to make sure my clients are 100% satisfied with their purchases and check emails/messages consistently throughout the day. You can rest assured that everything being sold here is as-described or your money back. I look forward to working with you!
Related Articles
Subscribe to get validation
news and free tips by email.
Need Additional Help?