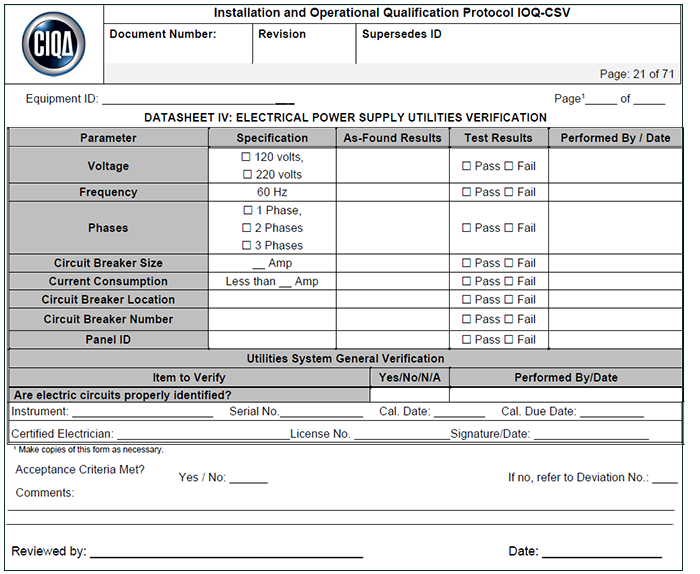
What are Test Scripts/Cases?
SUBSCRIBE AND FOLLOW US TO LEARN MORE ABOUT TEST SCRIPTS.For more details on specific FDA expectations and how to perform a Computer System Validation test script/case, follow us. Three (3) Options to Create a Test Scripts / Cases in Computer System Validation Protocol:Option 1. You Can Create Test Scripts in CSV Protocol, using a Template.You can download samples of the validation templates in .pdf format. To see the complete list of the most popular validation templates, click here. In addition, you can request a quotation to buy online a full validation template document in MS Word format that is completely editable, ready to fill and adapt to your needs. Option 2. We Can Bring You a Formal Training on How to build a Test Scripts in a Computer System Validation Using Our Template(s).This option is recommended if you want to learn more about how to build a robust validation protocol. One of our expert(s) will provide online step-by-step training to your team (unlimited assistance) on how to build a reliable validation protocol using a template. You can improve your corporate validation procedures and policies incorporating our template sections. It includes the template, an exam, and a training certificate for each assistant. Request a quote now. Option 3. We Can Create Customized Test Scripts and Computer System Validation Protocol.One of our expert(s) will create and prepare for you a customized validation protocol with the inputs and specific information of your company. It may include, online support in document creation, execution, or final reporting, Request a quote online. GET IN COMPLIANCE TODAY, CONTACT US (Hablamos Español)References:httpss://www.fda.gov/media/73141/download Food and Drug Administration References
Industry References
|

Ramon Cayuela, MS, BS, Chemical Engineering
CIQA President and CEO.
I've been working in validation engineering since 1992 with many multinational pharmaceutical companies. I love sharing my passion and knowledge with others. If you have any questions about anything (or just have general questions). I will be more than happy to assist you. You can count on the BEST customer service on CIQA. I go to great lengths to make sure my clients are 100% satisfied with their purchases and check emails/messages consistently throughout the day. You can rest assured that everything being sold here is as-described or your money back. I look forward to working with you!
Related Articles
Subscribe to get validation
news and free tips by email.
Need Additional Help?